A Cohesive Force Is Best Described as the ________.
This is the force that must be counteracted to increase the surface area. Force of attraction between molecules of the same substance is called cohesive force.

Cis 501 Midterm And Final Exam Strayer New
6916 6916 6916 The results are shown in Figure 3.

. The cohesive forces Van der Waal forces between these high molecular weight hydrocarbons is much less than that of water. A attraction between two liquid molecules. Water molecules at the surface are pulled into the liquid making the drop as small as possible.
The __________________ is a device used for cutting and attaching individual pieces of film. C force that allows for molecular cohesion. A strong adhesive force tends the liquid to spread over the surface whereas a strong cohesive force is responsible for the formation of water droplets on the water surface.
Cohesion force is present in between the water molecules. Attraction between molecules of two different substances. 7 A cohesive force is best described as the _____.
Jetc34 Jetc34 03042019 Physics High School answered Which of the following best describes a property of water. It has been suggested that compression force is dependent on the cohesive properties of a material. So from the given option the most relevant option going with the definition of cohesive forces is Forces of attraction between water molecules that forces present between the molecules of substance here water.
Cdominant over cohesive forces. Cohesion is the attraction of molecules in a liquid to each other due to inter-molecular forces. Adefinite volume and definite shape.
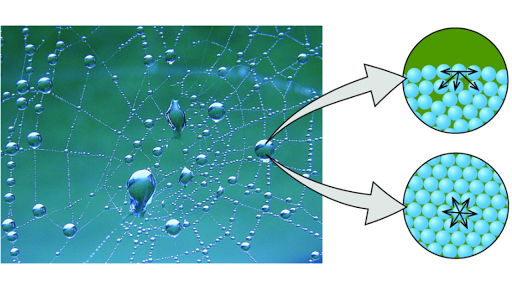
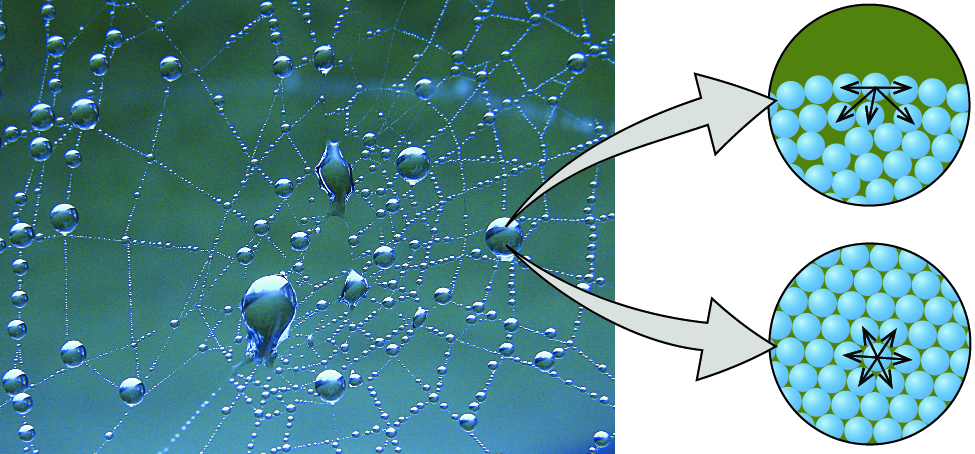
Cohesive is the adjective form of the verb cohere meaning to stick together. The net force also known as cohesion force on a surface molecule is a non-zero quantity in the direction towards the bulk Figure 21. Bdefinite volume but shape is determined by container.
D force that holds together the nucleus. B attraction between a liquid molecule and a solute. 6916 6916 6916 To validate this the tests were first replicated using the previously described methodology and products.
The force of attraction between molecules of unlike substances is called adhesive force. Surface tension is the. AWeak cohesive forces exist between its molecules.
It describes the property of one object tending to stick to adhere to another. A cohesive force is best described as the a attraction between molecules of two different substances battraction between a liquid molecule and a solute cforce that holds together. In physics cohesive describes the molecular force holding a body of similar parts together.
Which best describes the size and shape of a sample liquid. Adhesive force is the force of attraction between molecules of the different substances. Tendency for molecules at the surface of a liquid to hold together.
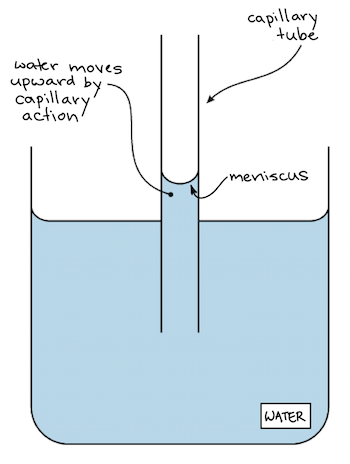
The effects of cohesion are capillary action meniscus and surface tension. As such improving the cohesiveness of internal teams should be a major goal for your organization. The key difference between cohesion and surface tension is that cohesion describes the intermolecular forces occurring between identical molecules whereas surface tension describes the elasticity of the surface of a liquid.
It is the force of the attraction that is present in the walls of the xylem and the water molecules. Roughly the same magnitude as cohesive forces. Comparatively the water has a greater affinity for itself so it beads.
B cohesive forces within water. Effective teamwork is the driving force behind most major business breakthroughs. The energy consumed by this process is called surface energy.
Define cohesive force and adhesive force of molecules. Attraction between a liquid molecule and a solute. C its nonpolar exterior.
Bvery weak compared to cohesive forces. The force of attraction that is present in between the two dissimilar substances is known as a cohesive force. A cohesive force is best described as the.
Cohesive forces are also known as intermolecular forces and can also be repulsion forces. Hydrogen bonding weak Van der Waals forces. This can be observe by over-filling liquid water into a glass.
Inter-molecular forces in between the molecules of the same substance like. The force of attraction between molecules of like substances is called adhesive force. Attraction between two liquid.
The liquid will stack on top of another until the inter-molecular force can no longer support it. The Five Behaviors profile system was developed by business management writer Patrick Lencioni to demonstrate how to build effective and cohesive teams. AWeak cohesive forces exist between its molecules.
Distinguish between adhesive and cohesive forces. When a glass surface is poured with water both adhesive and the cohesive forces act on the surface of the water. The adhesion and cohesion forces both vary in their strengths.
D all of the above. BIt cannot make many solutions CIt is a liquid over a wide temperature range. This causes a tendency in the liquids to avoid separation.
Cohesive force is the attractive force between like molecules. Surface tension is a property of liquids which arises due to the cohesion forces between identical liquid molecules.

Ap Chemistry Practice 3 Science Quizizz

Cis 501 Midterm And Final Exam Strayer New

Cohesion Force An Overview Sciencedirect Topics
Cohesion And Adhesion Of Water Article Khan Academy

Cis 501 Midterm And Final Exam Strayer New

In Water Hydrogen Bonds Are Best Described As A Cohesive Forces Between Water Brainly Com
Cohesion And Adhesion Of Water Article Khan Academy

Cis 501 Midterm And Final Exam Strayer New

Chem 180 Set 2 Flashcards Quizlet

It Project Man 600 Ca Test 2 Mcqs
Cohesion And Adhesion Of Water Article Khan Academy

Mcq On Surface Tension Free Pdf Yb Study
2 2 Water Concepts Of Biology 1st Canadian Edition

Intermolecular Forces And Adhesives

Cis 501 Midterm And Final Exam Strayer New

Attitude Discipline And Respect Ppt Download



Comments
Post a Comment